A common question asked to growers when troubleshooting a crop problem is, "What are you fertilizing with?" and "At what concentration is that being applied?" Greenhouse growers often know what ppm (parts per million) N (nitrogen) they are fertilizing at because they mix their fertilizer according to the chart on the fertilizer bag. Human error and problems with fertilizer injectors can affect concentration and a grower might not be fertigating to the level that they expect. But how can growers be sure the fertilizer concentration applied to the crop is the same as the intended concentration? Michigan State University Extension recommends checking the irrigation solution's EC (electrical conductivity) to help determine the actual ppm N in the crop’s irrigation water.
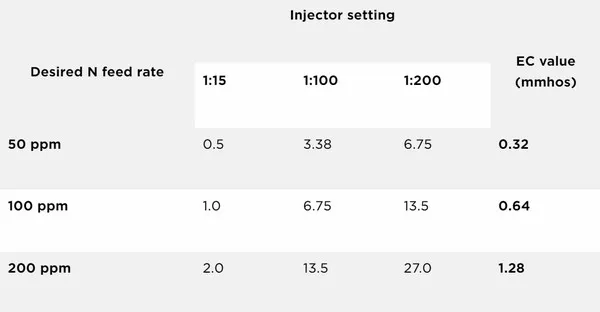 Table 1. A commercial water-soluble fertilizer label provides the amount of dry fertilizer (oz.) to add to each gallon of water to make a concentrated solution and the EC value for a given ppm N. Note: The EC listed is of the diluted solution after fertilizer is injected into clear water
Table 1. A commercial water-soluble fertilizer label provides the amount of dry fertilizer (oz.) to add to each gallon of water to make a concentrated solution and the EC value for a given ppm N. Note: The EC listed is of the diluted solution after fertilizer is injected into clear water
How to check fertilizer concentration
A high-quality water-soluble fertilizer will provide a chart indicating the EC value to the corresponding ppm N (Table 1). For example, in Table 1, when mixed at 100 ppm N, this fertilizer will have an EC value of 0.64 mmhos. It is important to note this is not the EC of the concentrated stock solution, but the EC of the diluted solution after fertilizer is injected into clear water. These values listed on the bag are the EC values when fertilizer is added to pure water. Since most growers are irrigating with city or well water (not water treated with reverse osmosis or distilled water), they need to account for the EC value of the irrigation water before fertilizer is added.
To test the EC of unfertilized irrigation water, fill a 5-gallon bucket with the same water used to dilute the fertilizer and measure its EC value. Take this number and subtract it from the EC value coming out of the “hose end” (irrigation water with fertilizer) and compare it to the chart on the fertilizer bag.

For example, suppose a grower's irrigation water with fertilizer had an EC of 0.78 mmhos, and their irrigation water without fertilizer had an EC value of 0.3 mmhos. In that case, they are actually fertilizing at approximately 75 ppm (0.78 EC of irrigation with fertilizer – 0.3 EC of irrigation with no fertilizer = 0.48 EC of fertilizer in irrigation water).
Although the chart in Table 1 does not specifically list 0.48 mmhos, growers can determine that it corresponds to a N concentration of 75 ppm because 0.48 is halfway between 0.32 and 0.64. When calibrating injectors, ensure that the EC at the hose end (water plus fertilizer) is equal to the EC of the clear water plus the EC for your target N rate from the bag.
Troubleshooting an incorrect concentration
In addition to checking the injector for mechanical issues, some other tips to ensure the correct concentration of fertilizer are to:
- Mix the concentrated stock solution with warm water to ensure the fertilizer dissolves completely.
- Do not make a stock solution at a concentration above the solubility listed on the label (Table 1).
- Double-check the stock tank to ensure the volume accurately matches the mixed ounces per gallon of fertilizer.
- Mix the fertilizer at the appropriate concentration for the target EC and injector ratio listed on the label. Every fertilizer will need different ounces per gallon to achieve the desired ppm N. Do not use the label from a different fertilizer to calculate another's concentration.
If growers have ruled out human error, the injector could be the culprit. Follow manufacturers' guidelines for regular injector cleaning and maintenance, as well as storage when not in use. Remember, running unfiltered fertilizer through an injector can cause it to clog or stick.
Source: canr.msu.edu
